Abstract
Background: Disease specific prognostic scores play a key role in risk-stratification and treatment decision-making process in Myelodysplastic Syndromes (MDS). An updated version of the International Prognostic Scoring System-Revised (IPSS-R), based on classical clinical variables-and molecular data (IPSS-M), has been recently proposed. Since some of the genes included in the IPSS-M are unfrequently mutated in MDS and, therefore, not commonly assessed in routine diagnostic laboratories (ex. MLL-PTD), we aimed to analyze the applicability of the new IPSS-M in a "real-life” setting.
Methods: We retrospectively collected clinical (including treatment), cytogenetic and molecular data from our real-life cohort of patients with MDS from a single institution. Inclusion criteria were MDS and MDS/MPN (< 13 x109/L leukocytes) diagnosed between 2018 and 2022 with molecular data from a commercial NGS panel containing myeloid-related genes. This panel comprises 27 out of 32 genes included in the IPSS-M, not analyzing MLL-PTD, BCORL1, ETNK1, GNB1, and PPM1D. Patients were classified according to IPSS-R and IPSS-M, and differences for risk stratification were evaluated in each patient to assess clinical implications of score-related variability.
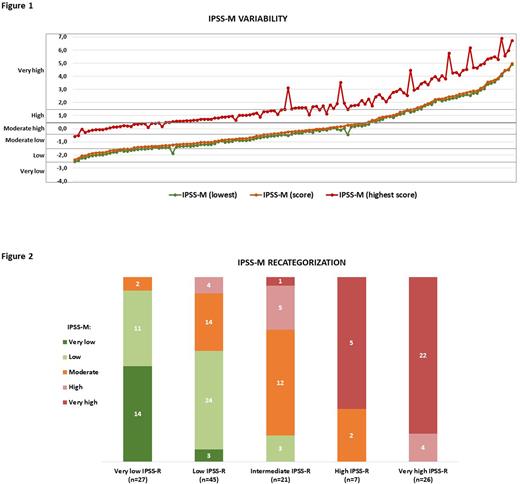
Results: From a total of 208 patients diagnosed with MDS during the study period, 126 (60.6%) patients were finally selected based on the availability of molecular data at the time of diagnosis. Despite the lack of 5 genes belonging to the IPSS-M, the IPSS-M calculator assigned a score for each patient as well as a range for best-worst potential scores. The mean difference between the assigned score and the lowest and highest ones was 0.15 (±0,8) and 1.75 (±0.37), respectively (Figure 1). The assigned score was closer to the best potential lowest score, due to the fact that mutations in the missing genes in our NGS panel are rarely mutated in MDS.
Overall, 42.9% (54/126) of the patients became reclassified to a different risk category when IPSS-M was calculated (Figure 2). However, in only 17.4% (22/126) of patients these changes were considered relevant from the therapeutic standpoint, namely patients considered as low risk who may have benefited from a more intensive treatment, and vice versa. Of these, 11.9% (15/126) cases were upgraded and 5.6% (7/126) were downgraded. Nonetheless, and after analyzing each patient individually following our institution's guidelines, only 12/126 (9.5%) of patients would have been managed differently if IPSS-M had been initially applied, mainly because part of the higher risk patients resulted not candidate to intensive care due to age and comorbidities.
Conclusions: According to our cohort of MDS patients, the proportion of cases who might benefit from the re-stratification according to the IPSS-M, in terms of potential differences in clinical management, is lower than initially expected. In spite of that, information derived from the NGS molecular should be the basis for different treatment approaches in the future.
FIGURE LEGENDS:
Figure 1. IPSS-M range variability. The y-axis represents the IPSS-M score value. Each patient is represented by 3 dots in the x-axis including a dot for the IPSS-M calculated score (orange) and the range from the lowest/best IPSS-M (green) and the highest/worst IPSS-M (red). Patients are ordered from lowest to highest IPSS-M calculated score.
Figure 2. IPSS-M recategorization. Each IPSS-R category in our cohort is represented by a bar, which is then color coded according to the number of patients that are re-classified according to the IPSS-M categories (moderate low and moderate high are merged into one category in IPSS-M).
Disclosures
Palomo:Janssen: Consultancy. Molero Yordi:Oryzon Genomics: Consultancy. Bosch:Karyospharm: Honoraria; Takeda: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Beigene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal